
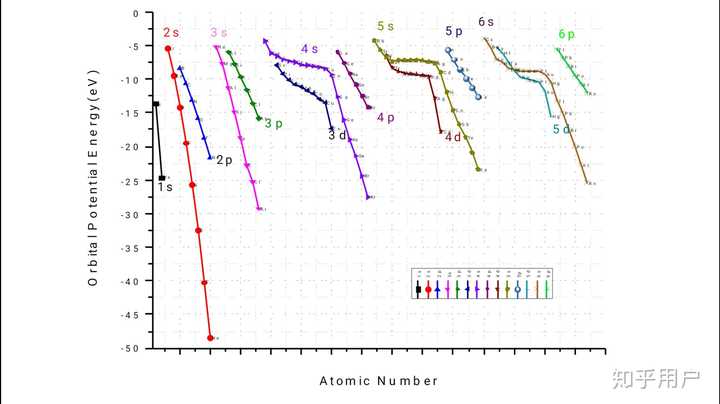
To take electron screening into account, the effective nuclear charge for the atomic orbitals should determined by Slater's rules.
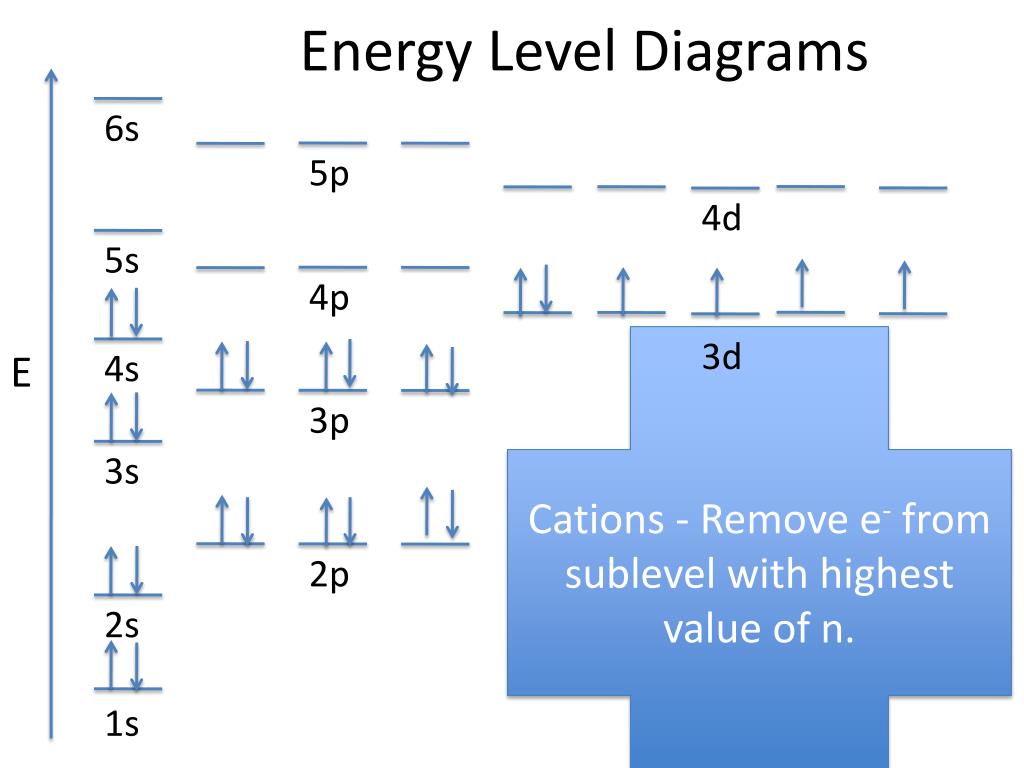
A reasonable first guess for the many-electron wave function for an atom is to construct a Slater determinant from the spin orbitals that make up that wave function. The first sum are the kinetic energies of the electrons, the second sum are the attractive Coulomb interactions between the electrons and the nucleus, the third sum are the repulsive electron-electron interactions.

The Hamiltonian for an atom with many electrons is,


 0 kommentar(er)
0 kommentar(er)
